Heterogeneous Electrocatalysis
Nanoparticle catalysts for electrochemistry
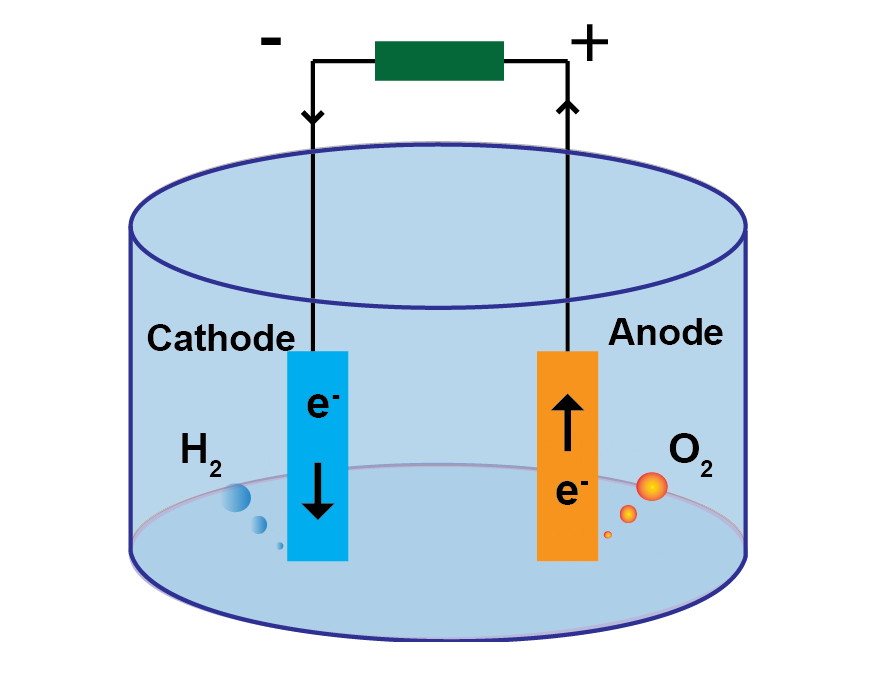
What is an electrochemical cell?
An electrochemical cell converts chemical energy into electrical energy or uses an applied electrical signal to trigger chemical reactions. The cell comprises an electrolyte and two electrodes. At the anode chemical species lose electrons and undergo oxidation reaction. At the cathode a reduction reaction occurs where the chemical species gains electrons. In a water splitting electrochemical cell an electrical current is applied to split water into oxygen and hydrogen via the reaction, 2H2O→ 2H2 + O2. The 2 half reactions occurring at each electrode are:
Oxygen Evolution Reaction (OER) at the Anode:
2H2O ⇔ O2 + 4H+ + 4e–
In order to reduce the energy barrier for a chemical reaction and increase the reaction efficiency, catalyst materials are commonly added to each electrode. The choice of catalyst is determined by the over potential for each reaction. Common catalyst materials are Platinum, Ruthenium, Iridium due to their chemical stability, corrosion resistance and high melting point
Electrochemical Applications
Green Hydrogen
water splitting
BioFuels
bioethanol, biodiesel
Fuel Cells
Hydrogen Fuel cells, Methanol Fuel cells
Carbon recycling
CO2RR, ethylene
Batteries
Lithium ion
Nanoparticle catalyst properties
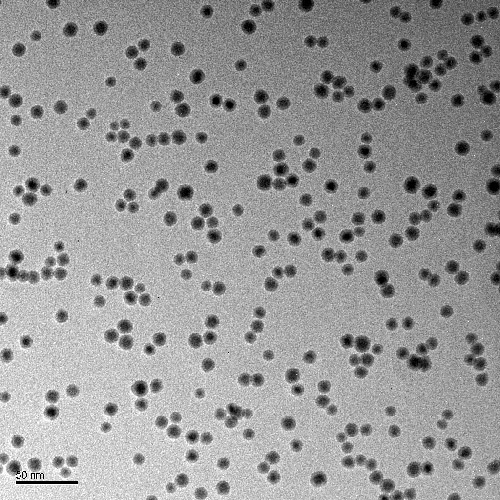
Nikalyte’s nanoparticles are ideal suited to electrocatalysis due to the precise control over nanoparticle properties such as size, structure and alloy composition. The nanoparticles are formed in vacuum from 99.00% pure solid source material, so there are no ligands or hydrocarbons on the surface, meaning that the nanoparticle surface is clean and able to take place in reactions.
Wide choice of catalytic materials
..including platinum, ruthenium, iridium, copper, and nickel.
Hydrocarbon Free
..generated in vacuum from 99.99% pure source material
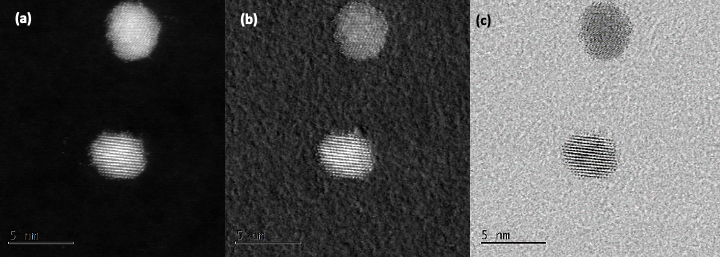
Crystalline
...crystalline structure.
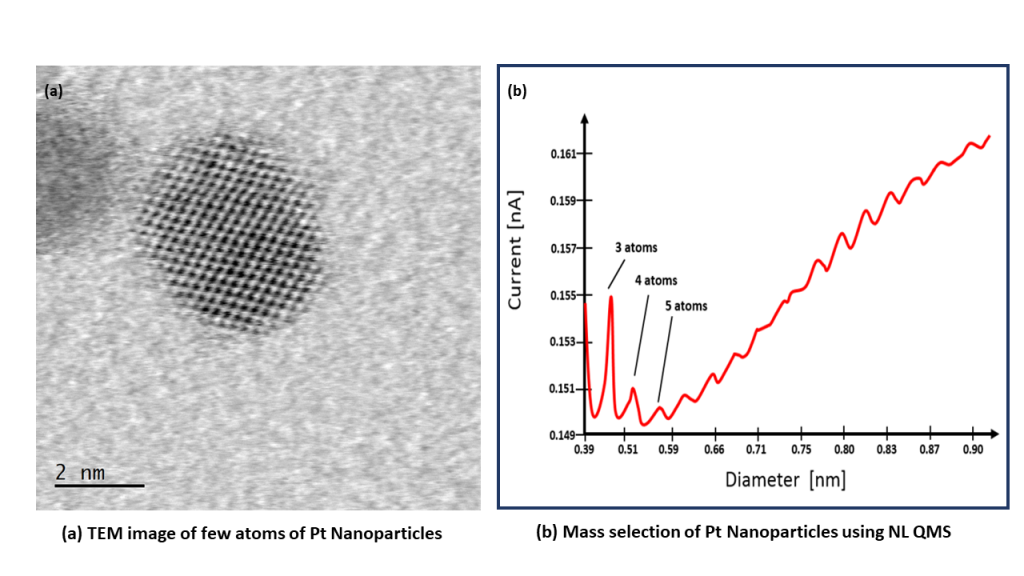
Control over nanoparticle size
..generate nanoparticles from 2-25nm with control over cluster size with NL-QMS
Materials for catalysis
Platinum
Platinum is still the material of choice as a catalyst for many electrochemical reactions, including the Hydrogen Evolution Reaction for water splitting. Platinum nanoparticles can be generated simply and quickly in the NL50 bench top deposition tool. The easy to use and compact NL50 has been designed specifically for the preparation of platinum group metals, including platinum, ruthenium and iridium. Deposit Platinum nanoparticles with controlled size using the NL-QMS mass filter
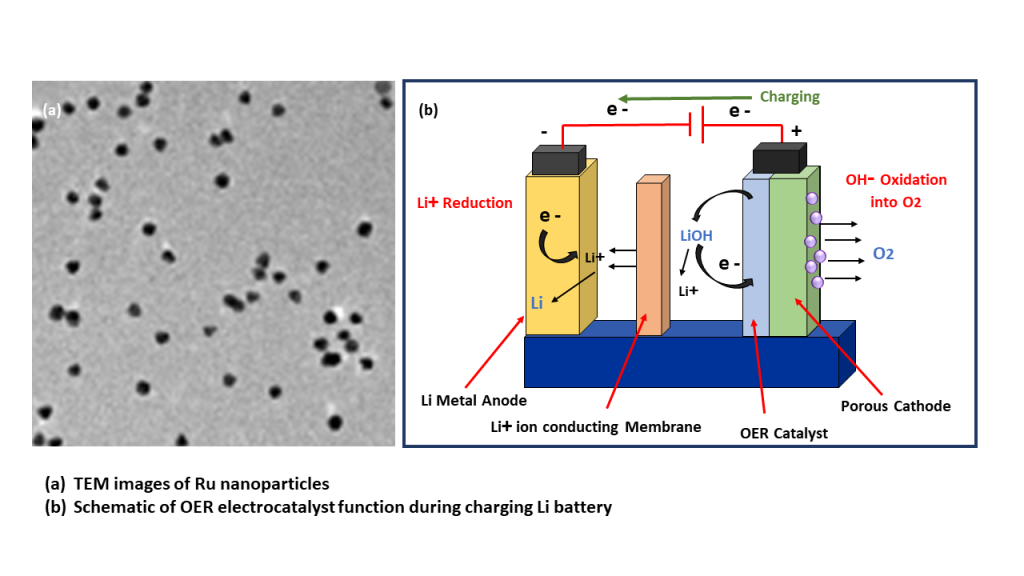
Ruthenium
To achieve cost effective hydrogen production, it is essential to have electrocatalysts that minimize energy wastage by reducing overpotentils in the Oxygen Evolution Reaction (OER). Additionally. Iriduim and Ruthenium are still the best performing OER catalysts due to their stability under aggressive conditions.
Copper
Copper oxide nanoparticle cathodes are considered to be highly efficient and selective catalysts for producing multi carbon products such as ethylene through the Carbon Dioxide Reduction Reaction (CO2RR). Deposit copper oxide nanoparticles using the NL50 nanoparticle deposition system with a single automated process with repeatable loading control.