Image Description: Ultra-sensitive NO2 MOF sensors designed for security and diagnostic applications.
Industry: Environmental Monitoring / Security / Sensor Technology
Challenge:
In work recently published, by Lister et al. from the Materials department at Oxford University researchers developed ultra-sensitive gas sensors capable of detecting trace concentrations of NO2 gas for applications in security, pollution monitoring, and medical diagnostics. Specifically, the challenge was to create high-sensitivity sensors for detecting harmful gases such as nitrogen dioxide (NO2) in parts-per-billion (ppb) concentrations. This required a breakthrough in sensor technology that could provide accurate and reliable measurements in real-time.
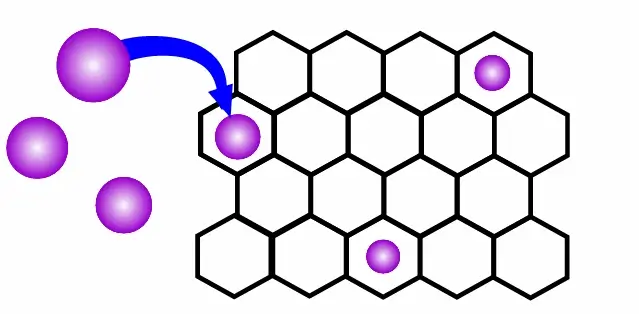
Image Description: Schematic showing gas molecules (purple) being captured in the pores of a 2D framework (black)
Solution:
Nikalyte’s NL50 Technology was used to develop Cu nanoparticles to seed metal-organic framework (MOF) growth. By depositing copper nanoclusters onto a patterned array of interdigitated electrodes, we facilitated the growth of conductive 2D MOFs in-situ during the electrochemical process. This electrochemical synthesis allowed for the formation of highly sensitive, ready-to-use sensors for detecting NO2 gas at very low concentrations.
The integration of electrically conductive MOFs enhanced the sensor’s sensitivity and response time, which are both key attributes for gas detection. When gases like NO2 are trapped in the porous structure of the MOF, it causes a measurable change in the electrical resistance of the material, which is used to quantify gas concentrations.
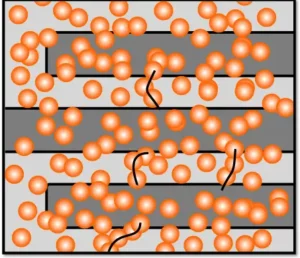
Image Description: Copper nanoclusters are deposited onto a pattern of interdigitated electrodes, forming isolated clusters between the gaps
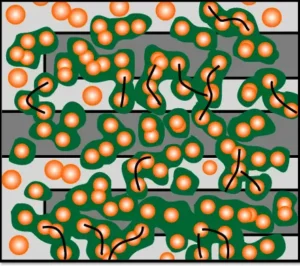
Image Description: After applying an electrical potential, conductive MOFs (green) grow from the nanoclusters, bridging the gaps (black lines) and creating new paths for current flow
Results:
The gas sensor was tested by exposing it to parts-per-billion (ppb) concentrations of NO2 gas for 60 seconds, during which the resistance of the sensor was measured. The sensor showed reversible sensing responses, with the magnitude of the response proportional to the gas concentration.
Key Findings:
- The limit of detection (LOD) for the sensor was calculated to be 64 ± 5 ppb for NO2, making it suitable for detecting trace amounts of NO2 gas for a variety of applications.
- The electrochemical growth of the Cu3(HHTP)2 MOF on the electrodes eliminated common issues like the coffee-ring effect and high contact resistance, which are often encountered in other synthesis methods.
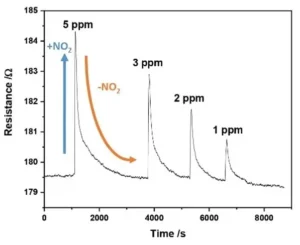
Image Description: Graph of sensor resistance over time (60s) as they are exposed to varying ppb concentrations of NO2 gas.
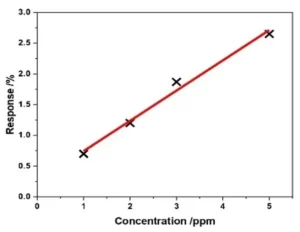
Image Description: Graph illustrating sensor responses that are reversible and proportional to gas concentration.
Conclusion:
This electrochemical synthesis method has enabled the creation of a high-performance gas sensor capable of detecting NO2 in the ppb range, which can be applied in fields such as security (e.g., trace explosive detection), environmental pollution monitoring, and medical diagnostics (e.g., exhaled breath analysis for diseases like diabetes). The in-situ formation of electrically conductive MOFs on the electrodes presents a promising route for low-cost, highly sensitive gas sensors in various industries.

Note: Full details of the project and results can be found in the paper here
Looking to develop high-sensitivity sensors for your environmental or security applications?
Contact us today to learn more about how Nikalyte’s nanoparticle equipment can elevate MOF-based technology to achieve superior gas detection.

