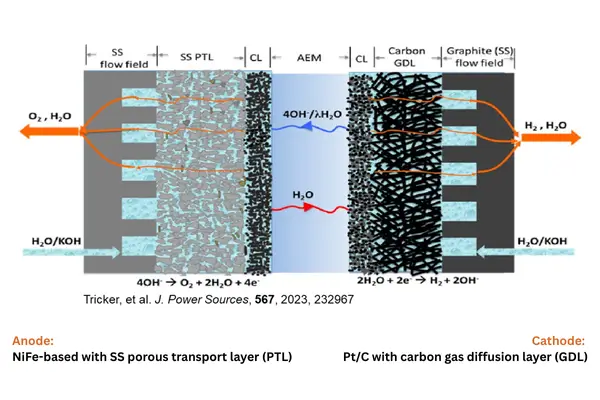
Image Description: Schematic of an AEMWE (Alkaline Anion Exchange Membrane Water Electrolyzer) cell, illustrating the different layers involved in the electrolysis process.
Industry: Renewable Energy / Electrochemical Research / Hydrogen Production
Challenge:
The transition to net-zero emissions requires efficient, scalable methods for hydrogen production, such as water electrolysis. A significant challenge in this area is the development of low-cost, earth-abundant electrocatalysts that can perform well in real-world conditions, such as alkaline anion exchange membrane water electrolysers (AEM-WEs).
Existing iridium-based catalysts, while effective, are expensive and scarce, limiting the scalability of hydrogen production. Transition metal-based (TM-based) catalysts offer a more affordable and sustainable alternative, but they often lack stability in acidic electrolytes. Moreover, laboratory-scale catalyst screening methods do not always translate to real-world electrolysis performance, leading to discrepancies between laboratory results and industrial applications.
This project, involving the collaboration of University of Oxford, Manchester Metropolitan University, Nikalyte Ltd, , and the National Physical Laboratory (NPL), aimed to address these challenges by benchmarking electrocatalysts and refining testing methods for consistent, real-world performance.
Solution:
Nikalyte’s NL-UHV nanoparticle deposition Technology played a pivotal role in this project by facilitating the deposition of NiFe2O4 nanoparticles onto various electrode formats. This technology enabled the precise and reproducible preparation of electrode surfaces, which are crucial for evaluating the performance of electrocatalysts in real-world electrolyser setups.
The project focused on transition metal-based catalysts such as NiFe2O4, chosen for its promise in alkaline media. The research also employed novel testing techniques, such as the floating electrode method, to more accurately evaluate the catalysts in conditions that mirror actual electrolyser environments. This approach allowed for the rapid screening of catalysts, including NiFe2O4 and other Ni-based alternatives, directly compared with the benchmark IrOx catalyst.
Key insights from the project showed how electrode geometry, electrolyte flow rates, and other factors impact catalytic performance, leading to the establishment of a standardized protocol for consistent catalyst testing across different labs.
Image Description: Top-down view of a catalyst-coated membrane, showing the anode side after soaking in KOH prior to assembly (image credit Manchester Metropolitan University).
Results:
Through a series of rigorous tests and collaborations between academic and industrial partners, several critical findings emerged:
- NiFe2O4 Catalyst Performance: The electrochemical properties of NiFe2O4 were benchmarked against IrOx, and the results showed promising potential for alkaline electrolysis applications.
- Improved Testing Protocols: Variations in electrolyser geometries, electrolyte temperature, and other factors were shown to significantly influence performance. The project led to the development of a standardized testing protocol for OER catalysts, ensuring consistent results across labs.
- Floating Electrode Method: The first-ever application of this method in alkaline media revealed its potential for faster, more accurate catalyst screening. This method can be adapted for various TM-based catalysts, paving the way for rapid innovation in electrocatalyst development.
Key Findings:
- The new testing protocol enhanced reproducibility across labs and demonstrated the significant influence of electrode preparation on catalyst performance.
- The floating electrode method proved to be a reliable tool for screening low-cost, earth-abundant catalysts such as NiFe2O4.
Conclusion:
This project has advanced the development of low-cost, earth-abundant electrocatalysts for water electrolysis, contributing valuable insights to the field of sustainable hydrogen production. The standardized testing protocols and the introduction of the floating electrode method are key innovations that can accelerate the development of scalable hydrogen technologies.
The collaboration between academic institutions and industry partners, supported by Royce’s Industrial Collaboration Programme, has set the stage for further research into optimizing catalyst adhesion, improving electrode preparation methods, and scaling up hydrogen production for net-zero solutions.
Note: Full details of the project and results are confidential, protected under an NDA.
Looking to develop efficient electrocatalysts for your hydrogen production system?
Contact us today to learn more about how Nikalyte’s nanoparticle deposition technology can advance your electrochemical research and help scale up sustainable hydrogen production.