Entropy is a complex concept. It is usually associated with disorder or randomness. Yet this is a holdover from classical thermodynamics. In the context of catalysts, entropy is better described as complexity. High-entropy alloys (HEAs) thus feature elaborate and highly configurational microstructures. This results from their diverse heterogeneity: HEAs contain no less than five elements in roughly equal amounts. Scientists can adjust these many components to achieve fine-tuned properties. High hardness, strength, wear resistance, thermal stability, corrosion resistance, and more. High-entropy alloys can yield many desired results. This represents enormous potential in next-generation catalysis.
Multielemental Active Sites: A Game-Changer
One of the most compelling attributes of high entropy alloy catalysts is their ability to offer many multielement active sites on a singular catalytic surface. This diversity in active sites can significantly enhance catalytic activity. Unlike traditional catalysts, which often have limited active sites, these alloys provide a broader spectrum of reactivity.
The Role of High Entropy in Catalysis
Entropy, in the context of these alloys, is not just a buzzword. The high entropy intrinsic to these materials leads to a more complex structure. This complexity can amplify the number of active sites available, thereby optimising catalytic performance. It’s akin to having more hands on deck, ready to facilitate reactions.
The Cocktail Effect: Synergy in Action
Beyond individual active sites, the “cocktail effect” of high entropy alloy catalysts is a phenomenon worth noting. This refers to the synergistic effect of multiple elements present in the alloy. It’s not just about having multiple elements; it’s about how they work in tandem to boost catalytic activity. This synergy can often lead to outcomes that are greater than the sum of individual contributions.
Sustainability: The Future of Catalysis
In an era where sustainability is no longer a luxury but a necessity, high entropy alloy catalysts represent a promising investment. Their potential applications in carbon dioxide reduction, water electrocatalysis, and oxygen reduction underscore their role in fostering a more sustainable future. HEAs offer a cheaper and more earth abundant alternative to iridium catalysts currently used to produce hydrogen through water splitting. By leveraging these alloys, we can inch closer to eco-friendly solutions that address pressing global challenges.
Interested in High Entropy Alloy Research?
At Nikalyte, we are well-acquainted with the potential of high entropy alloy catalysts. With our expertise in nanoparticle deposition, we have developed a unique multi elemental magnetron sputtering solution that lends itself to HEA generation.
Fig1. TEM images HEA nanoparticles generated by Nikalyte, comprising (a) NiFeCoMoCr and (b) NiFeCoMoAl
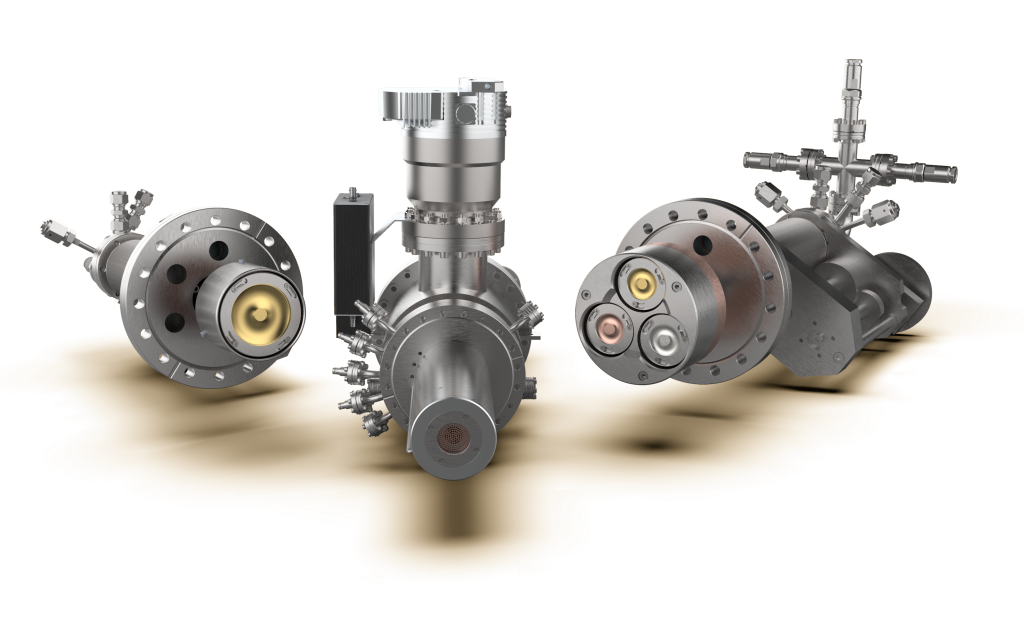
The Nikalyte NL-UHV system is designed for generating and depositing nanoparticles in an Ultra-High vacuum environment. This instrument offers control over the nanoparticles’ size, composition, and structure, allowing for direct deposition onto various substrates and materials. The resulting films can be density adjusted and adhere to the sample surface.
The NL-DX3 variant of the NL-UHV is ideally suitable for research involving HEAs. It features a triple 1” source, enabling loading up to three different binary alloys. This provides the option to deposit materials individually or in combinations of two or three, offering flexibility in alloy composition.
The Nikalyte NL-QMS mass filter is also available for those seeking enhanced control over nanoparticle deposition. This tool allows for the real-time analysis of deposited nanoparticles by mass or diameter. The NL-QMS is conveniently controlled through WindowsTM software.
The NL-UHV and NL-DX3 offer a platform for studying and utilising various catalytic materials. The Nikalyte range aims to provide precision and efficiency in nanoparticle deposition tasks.