Hydrogen production has emerged as a key player in the race to achieve net zero emissions, offering a clean and efficient energy source with near-limitless applicability. Yet the task of decarbonizing power generation in-line with the United Nations’ Net Zero Coalition remains formidable.
The Hydrogen Council estimates that 75 metric tons (MT) of clean hydrogen is needed by 2030, and that renewable and low-carbon hydrogen could feasibly abate 80 gigatons of carbon dioxide (CO2) by 2050. That may sound optimistic, but total installed electrolysis capacity dedicated to hydrogen production reached more than 200 MW in 2021–a threefold increase on the previous year. Thanks to this impressive growth, the total installed capacity of green hydrogen production has now reached 0.5 GW and was projected to exceed 1 GW by the end of 2022. If projections are accurate, and all planned projects reach fruition, installed electrolyser capacity could reach 240 GW by 2030. That’s twice what experts predicted the previous year.
However, expectations should be tempered, as there are enduring challenges in expanding electrolysis capacity to meet demand.
Using Nanoparticle Technology to Drive Hydrogen Production
Catalytic electrolysis is the bedrock of hydrogen production. When coupled with sustainable energy sources, it is the go-to formulation method for green hydrogen manufacturing. Although significant investment is needed to realise the true environmental benefits of the green hydrogen value chain, some estimates suggest these investments will create a self-sustaining market worth $2.5 trillion by 2050. Many experts are turning to the nanoscale to help realise this vision.
Electrolysis uses electricity to split water molecules into hydrogen and oxygen, by passing a current through an electrolytic solution (i.e. sodium hydroxide). The current splits water into its components, which are attracted to either an anode or a cathode. Manufacturers can drive efficiency further by using a catalyst to reduce the activation energy. Catalyst efficiency can likewise be enhanced depending on its production method. Manipulating the size, shape, and composition of nanoparticles allows manufacturers to produce extremely efficient and selective catalysts with unmatched surface properties. But this doesn’t always resolve fundamental issues like exorbitant raw material costs or scarcity.
Common catalysts for hydrogen production include platinum combined with iron and nickel hydroxide, alongside other expensive noble metals like iridium. Others include ruthenium, which is an extremely versatile catalyst with high intrinsic catalytic activity, enhanced thermal stability, and excellent catalytic performance in oxidation reactions. The catch with ruthenium is its scarcity. Nevertheless, researchers globally have long been exploring novel catalyst formulations which exhibit synergistic effects. Researchers at Pohang University created a double-layered catalyst composed of a platinum nanoplate flattened over nickel-iron layered doubled hydroxide. The result displayed 11.2 times higher activity than conventional catalysts.
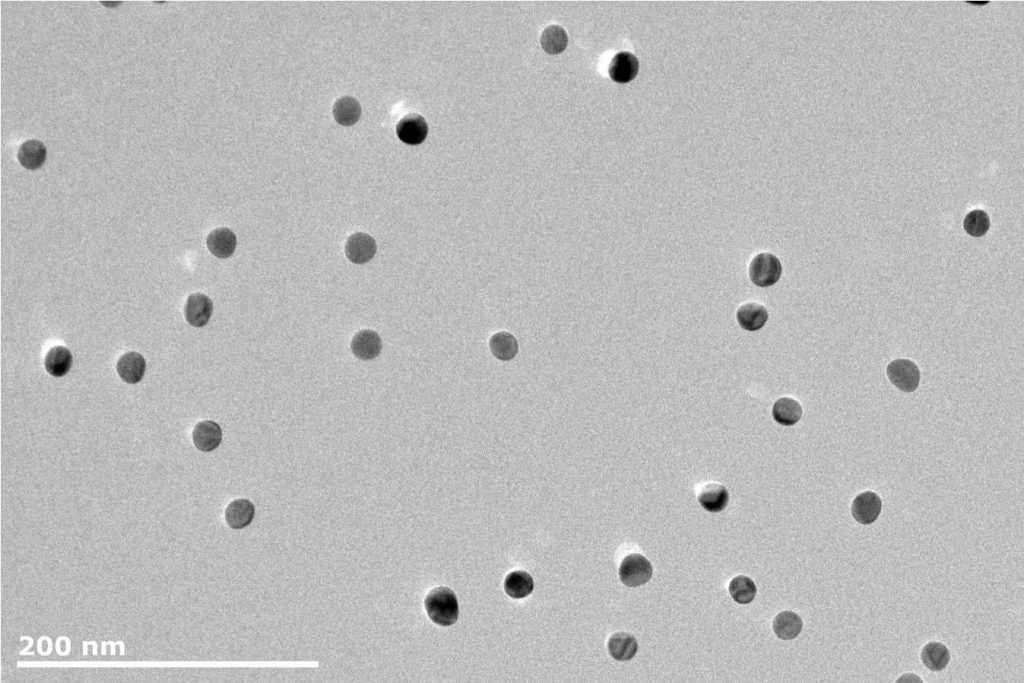
Using Core-Shell Structures to Optimize Catalytic Activity
Core-shell structures are a similar approach to optimizing catalytic activity by maximizing the surface area of active material. Sometimes known as nanocomposites, core-shell catalysts are nanoparticles comprising an inner core that acts as a substrate for the active material, maximizing the surface area for optimal activity. Research into this area is ongoing, but results are promising. The key to realising the benefits of core-shell nanocatalysts is producing active surfaces of subnanometer thickness on a functional core, which requires a reliable synthesis method.
The NL-DX3 is a versatile deposition source that enables loading of up to three separate materials, providing increased opportunities to form stable core-shell structures as each material can be controlled independently. Up to three materials can be deposited individually, or as alloys of two or three materials simultaneously. This provides incredible scope for producing core-shell structures. The NL-DX3 system is designed to deposit ultra-pure metallic or compound nanoparticles (including oxides or nitrides) onto samples, with control, and flexibility in mind. The vacuum deposition process ensures that the nanoparticles are free from hydrocarbons or other contamination commonly associated with chemical techniques. Samples can be plasma cleaned prior to deposition, and the nanoparticle deposition can be combined with other standard vacuum techniques, such as sputtering to generate complex structures.
Want to learn more? Contact a member of the team today.
References
- https://www.iea.org/reports/net-zero-by-2050
- https://www.iea.org/reports/electrolysers
- https://hydrogencouncil.com/wp-content/uploads/2021/11/Hydrogen-for-Net-Zero.pdf
- https://www.mckinsey.com/industries/automotive-and-assembly/our-insights/hydrogen-the-next-wave-for-electric-vehicles
- https://www.sciencedaily.com/releases/2022/06/220614122719.htm
- https://pubmed.ncbi.nlm.nih.gov/24045405/